Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Aug 20, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 511:
Morphine alkaloids derived from the opium poppy have long been used as analgesics. Codeine, the methyl ether of morphine, is a naturally occurring alkaloid with medicinal properties very similar to those of morphine. Thousands of derivatives of morphine have been synthesized and tested for their biological effects. For example, the diacylated derivative of morphine, heroin, is a highly addictive drug. Much effort has gone into understanding how morphine and its derivatives function.
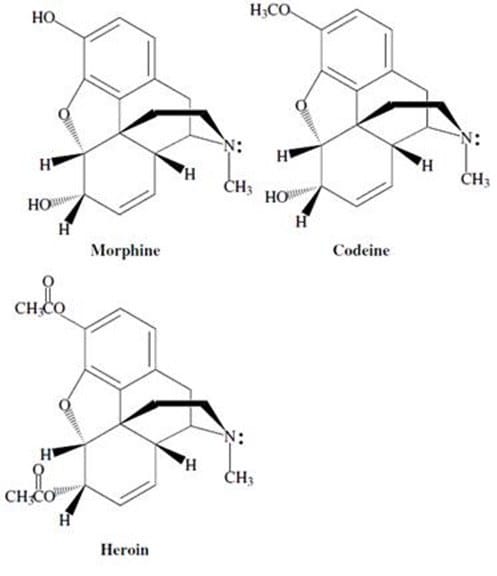
Studies have shown that certain common structural features of alkaloids are required for the compound to exhibit biological activity. These structural requirements are summarized by the so called "morphine rule":
Demerol and methadone, shown in Figure 2, are two synthetic alkaloids designed to satisfy the "morphine rule." Synthetic alkaloids such as these have been found to mimic certain physiological properties of morphine and its derivatives, and
have found pharmacological application due to other, more desirable biological effects. Methadone has been used widely in the United States and Great Britain as a treatment for heroin addiction; it reduces the physical symptoms
accompanying withdrawal without producing many of the other effects of heroin.
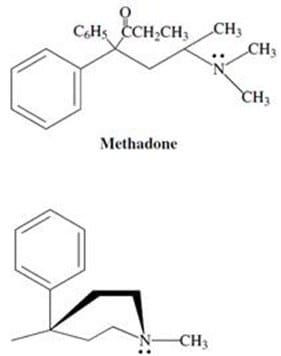
Meperidine (demerol) Figure 2
One of the requirements of the "morphine rule" is that an aromatic ring be attached to a quaternary carbon in order for the molecule to be biologically active. The quaternary carbon of any morphine-like substance must be:
I. a stereocenter
II. sp3 hybridized
III.
sp2 hybridized
A.
I only
B.
II only
C.
I and II only
D.
I and III only
-
Question 512:
In 1972, Georges Ungar reported the discovery of a peptide that appeared to transfer learning. Ungar's claim was based on experiments in which rats placed in a chamber with specially designed dark and light regions were trained to avoid
the dark regions of the chamber. Following their training, the rats were killed and brain extracts were prepared. These brain extracts were injected into naive rats which were then observed to acquire the fear of darkness without training. Two
hypotheses were proposed to explain these remarkable results:
Hypothesis 1
Ungar concluded that the extracts contained some chemical that transmitted the learned fear of darkness to the naive rats. A fifteen amino-acid polypeptide was isolated from the brain extracts and sequenced. Ungar claimed that this peptide,
called scotophobin, was a chemical transmitter of learning. The peptide had the primary structure shown below:
C-ser-asp-asn-arg-gln-gln-gly-lys-ser-ala-arg-gln-glygly-tyr-N scotophobin
Hypothesis 2
Other researchers, who tested scotophobin but could not reproduce Ungar's results, argued that scotophobin did not transfer the learned fear of darkness. Instead, they suggested that scotophobin, which is structurally similar to ACTH and
vasopressin, acted to increase stress in the rats. Since stress increases sympathetic nervous activity, rats injected with scotophobin would become hyperactive and tend to spend less time in the dark regions of the experimental chamber.
They argued that such stress responses in the rats could be misinterpreted as a fear of darkness. Ungar's claim was further weakened by chemical analysis in which both the scotophobin extracts which Ungar had injected into the naive rats
and a sample of synthesized scotophobin peptide were subjected to SDS polyacrylamide gel electrophoresis, as shown in Figure 1.
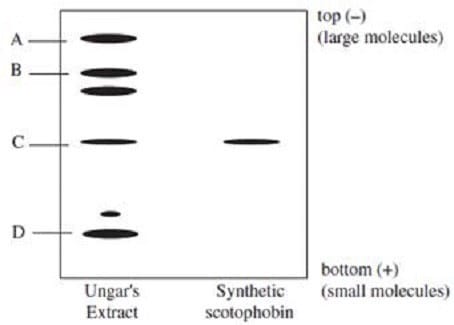
Figure 1
The chemical analysis of Ungar's extract most likely weakened his claim that scotophobin transferred the learned fear of darkness because it showed that:
A. the extract was not pure scotophobin.
B. the extract did not contain scotophobin.
C. scotophobin was not present in sufficient concentrations to illicit a response.
D. scotophobin was similar in structure to other proteins.
-
Question 513:
In 1972, Georges Ungar reported the discovery of a peptide that appeared to transfer learning. Ungar's claim was based on experiments in which rats placed in a chamber with specially designed dark and light regions were trained to avoid
the dark regions of the chamber. Following their training, the rats were killed and brain extracts were prepared. These brain extracts were injected into naive rats which were then observed to acquire the fear of darkness without training. Two
hypotheses were proposed to explain these remarkable results:
Hypothesis 1
Ungar concluded that the extracts contained some chemical that transmitted the learned fear of darkness to the naive rats. A fifteen amino-acid polypeptide was isolated from the brain extracts and sequenced. Ungar claimed that this peptide,
called scotophobin, was a chemical transmitter of learning. The peptide had the primary structure shown below:
C-ser-asp-asn-arg-gln-gln-gly-lys-ser-ala-arg-gln-glygly-tyr-N Scotophobin
Hypothesis 2
Other researchers, who tested scotophobin but could not reproduce Ungar's results, argued that scotophobin did not transfer the learned fear of darkness. Instead, they suggested that scotophobin, which is structurally similar to ACTH and
vasopressin, acted to increase stress in the rats. Since stress increases sympathetic nervous activity, rats injected with scotophobin would become hyperactive and tend to spend less time in the dark regions of the experimental chamber.
They argued that such stress responses in the rats could be misinterpreted as a fear of darkness. Ungar's claim was further weakened by chemical analysis in which both the scotophobin extracts which Ungar had injected into the naive rats
and a sample of synthesized scotophobin peptide were subjected to SDS polyacrylamide gel electrophoresis, as shown in Figure 1.

Figure 1
Researchers were interested in purifying a second protein (protein X) from Ungar's extract. The gene segment encoding protein X was believed to consist of thirty nucleotides. According to Figure 1, which band could represent protein X?
A. Band A
B. Band B
C. Band C
D. Band D
-
Question 514:
In 1972, Georges Ungar reported the discovery of a peptide that appeared to transfer learning. Ungar's claim was based on experiments in which rats placed in a chamber with specially designed dark and light regions were trained to avoid
the dark regions of the chamber. Following their training, the rats were killed and brain extracts were prepared. These brain extracts were injected into naive rats which were then observed to acquire the fear of darkness without training. Two
hypotheses were proposed to explain these remarkable results:
Hypothesis 1
Ungar concluded that the extracts contained some chemical that transmitted the learned fear of darkness to the naive rats. A fifteen amino-acid polypeptide was isolated from the brain extracts and sequenced. Ungar claimed that this peptide,
called scotophobin, was a chemical transmitter of learning. The peptide had the primary structure shown below:
C-ser-asp-asn-arg-gln-gln-gly-lys-ser-ala-arg-gln-glygly-tyr-N Scotophobin
Hypothesis 2
Other researchers, who tested scotophobin but could not reproduce Ungar's results, argued that scotophobin did not transfer the learned fear of darkness. Instead, they suggested that scotophobin, which is structurally similar to ACTH and
vasopressin, acted to increase stress in the rats. Since stress increases sympathetic nervous activity, rats injected with scotophobin would become hyperactive and tend to spend less time in the dark regions of the experimental chamber.
They argued that such stress responses in the rats could be misinterpreted as a fear of darkness. Ungar's claim was further weakened by chemical analysis in which both the scotophobin extracts which Ungar had injected into the naive rats
and a sample of synthesized scotophobin peptide were subjected to SDS polyacrylamide gel electrophoresis, as shown in Figure 1.

Figure 1
Researchers isolated a polypeptide from the brains of goldfish trained to avoid darkness. This goldfish scotophobin was 15 amino acids long and differed from rat scotophobin by one amino acid. The gene for goldfish scotophobin must differ from that of rat scotophobin by:
A. one amino acid.
B. three codons.
C. at least one nucleotide.
D. at least three nucleotides.
-
Question 515:
In 1972, Georges Ungar reported the discovery of a peptide that appeared to transfer learning. Ungar's claim was based on experiments in which rats placed in a chamber with specially designed dark and light regions were trained to avoid
the dark regions of the chamber. Following their training, the rats were killed and brain extracts were prepared. These brain extracts were injected into naive rats which were then observed to acquire the fear of darkness without training. Two
hypotheses were proposed to explain these remarkable results:
Hypothesis 1
Ungar concluded that the extracts contained some chemical that transmitted the learned fear of darkness to the naive rats. A fifteen amino-acid polypeptide was isolated from the brain extracts and sequenced. Ungar claimed that this peptide,
called scotophobin, was a chemical transmitter of learning. The peptide had the primary structure shown below:
C-ser-asp-asn-arg-gln-gln-gly-lys-ser-ala-arg-gln-glygly-tyr-N scotophobin
Hypothesis 2
Other researchers, who tested scotophobin but could not reproduce Ungar's results, argued that scotophobin did not transfer the learned fear of darkness. Instead, they suggested that scotophobin, which is structurally similar to ACTH and
vasopressin, acted to increase stress in the rats. Since stress increases sympathetic nervous activity, rats injected with scotophobin would become hyperactive and tend to spend less time in the dark regions of the experimental chamber.
They argued that such stress responses in the rats could be misinterpreted as a fear of darkness. Ungar's claim was further weakened by chemical analysis in which both the scotophobin extracts which Ungar had injected into the naive rats
and a sample of synthesized scotophobin peptide were subjected to SDS polyacrylamide gel electrophoresis, as shown in Figure 1.

Figure 1 In a follow-up experiment, researchers administered scotophobin to rats in order to examine its physiological effects. Which of the following observations of physiological effects in the rat would provide the best support for Hypothesis 2?
A. Increase in urine volume
B. The rat pupils dilate causing the rat to prefer darkness and avoid light
C. Increase in heart rate
D. Dilation of arterioles regulating blood flow to the digestive tract
-
Question 516:
In 1972, Georges Ungar reported the discovery of a peptide that appeared to transfer learning. Ungar's claim was based on experiments in which rats placed in a chamber with specially designed dark and light regions were trained to avoid
the dark regions of the chamber. Following their training, the rats were killed and brain extracts were prepared. These brain extracts were injected into naive rats which were then observed to acquire the fear of darkness without training. Two
hypotheses were proposed to explain these remarkable results:
Hypothesis 1
Ungar concluded that the extracts contained some chemical that transmitted the learned fear of darkness to the naive rats. A fifteen amino-acid polypeptide was isolated from the brain extracts and sequenced. Ungar claimed that this peptide,
called scotophobin, was a chemical transmitter of learning. The peptide had the primary structure shown below:
C-ser-asp-asn-arg-gln-gln-gly-lys-ser-ala-arg-gln-glygly-tyr-N scotophobin
Hypothesis 2
Other researchers, who tested scotophobin but could not reproduce Ungar's results, argued that scotophobin did not transfer the learned fear of darkness. Instead, they suggested that scotophobin, which is structurally similar to ACTH and
vasopressin, acted to increase stress in the rats. Since stress increases sympathetic nervous activity, rats injected with scotophobin would become hyperactive and tend to spend less time in the dark regions of the experimental chamber.
They argued that such stress responses in the rats could be misinterpreted as a fear of darkness. Ungar's claim was further weakened by chemical analysis in which both the scotophobin extracts which Ungar had injected into the naive rats
and a sample of synthesized scotophobin peptide were subjected to SDS polyacrylamide gel electrophoresis, as shown in Figure 1.
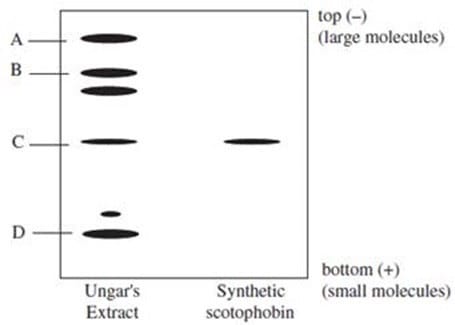
Figure 1
Hydrolytic enzymes cleave polypeptides at specific amino acid residues. Which of the following hydrolytic enzymes could be used to cleave scotophobin into three fragments?
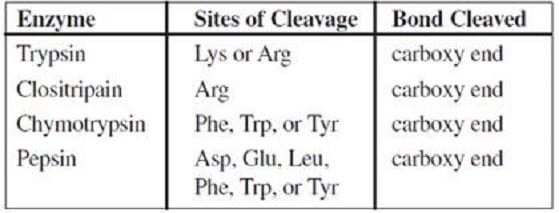
A. Trypsin only
B. Trypsin or clositripain only
C. Clositripain or chymotrypsin only
D. Clositripain or pepsin only
-
Question 517:
Which of the following functional groups are NOT found on the side chains of any of the naturally occurring amino acids?
A. Hydroxyl
B. Methyl
C. Carboxylic acid
D. Aldehyde
-
Question 518:
When humans are submerged in water, the mammalian dive reflex acts to alter circulation. Heart rate decreases, blood flow to the extremities is reduced, and mean arterial blood pressure is increased. These accomodations lead to:
A. decreased oxygen demand by the tissues.
B. increased heat retention by the body.
C. decreased partial pressure of oxygen in the blood.
D. increased venous return.
-
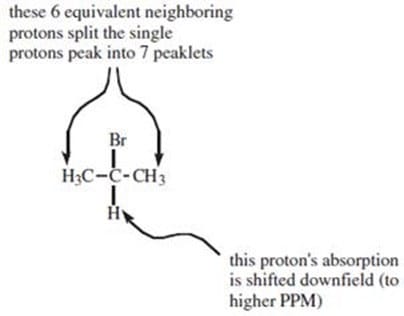
Question 519:
Which of the following molecules would produce the NMR spectrum shown below?
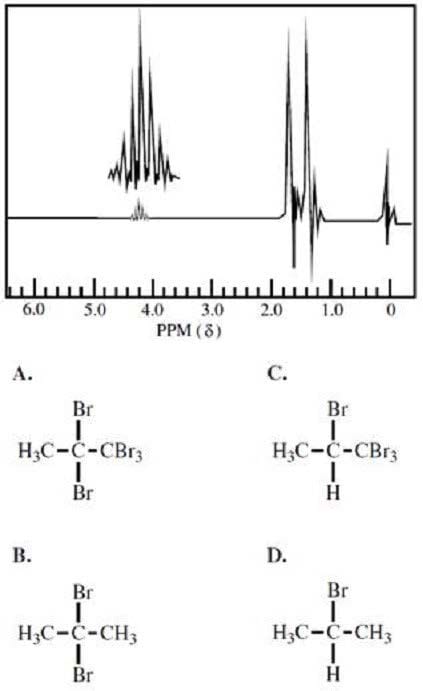
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 520:
Which of the following best illustrates the contracted state of the sarcomere shown below?
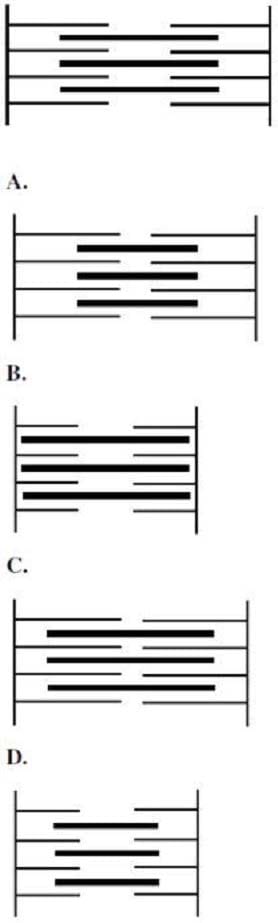
A. Option A
B. Option B
C. Option C
D. Option D
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.