Exam Details
Exam Code
:OATExam Name
:Optometry AdmissionCertification
:Test Prep CertificationsVendor
:Test PrepTotal Questions
:274 Q&AsLast Updated
:Aug 10, 2025
Test Prep Test Prep Certifications OAT Questions & Answers
-
Question 31:
For the following nuclear reaction, fill in the correct daughter product.

A. Option A
B. Option B
C. Option C
D. Option D
E. Option E
-
Question 32:
In ideal gas circumstances, if the pressure and volume were both doubled. What would happen to the temperature?
A. It would stay the same.
B. It would half.
C. It would double.
D. It would quadruple.
E. Cannot be calculated with this information.
-
Question 33:
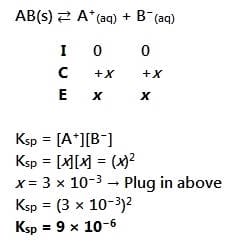
The molar solubility of an hypothetical salt, AB, is 3.0 × 10−3. Find the Ksp
A. 27 × 10−9
B. 9 × 10−6
C. 6 × 10−3
D. 1.5 × 10−3
E. 3.0 × 10−3
-
Question 34:
Which of the following is defined by the 2nd law of thermodynamics?
A. In any spontaneous process, the entropy of the universe always increases.
B. Energy cannot be created or destroyed.
C. The entropy of a perfect crystal approaches 0 as temperature approaches absolute 0.
D. Any object in motion will remain in motion unless a force is acted upon it.
E. For every action, there is an equal and opposite reaction.
-
Question 35:
All of the following are characteristics of a galvanic cell EXCEPT:
A. It is spontaneous.
B. Anode is the site of oxidation.
C. Anions flow to the anode via the salt bridge.
D. The cathode is negative.
E. All of the above are true.
-
Question 36:
A reaction with a positive S and a positive H is spontaneous when?
A. At all temperatures
B. At high temperatures
C. At low temperatures
D. The reaction is not spontaneous at all.
E. Not enough information is provided to complete this problem.
-
Question 37:
A titration of NaOH and HF is made. Which of the following equivalence points pH can be possible?
A. 7.0
B. 13.5
C. 1.2
D. 4.5
E. 9.3
-
Question 38:
Given the following reaction, how might one shift the equilibrium to the left? N2(g) + 3H2(g) <...> 2NH3(g)
A. Add N2(g)
B. Add helium
C. Decrease the pressure
D. Remove NH3(g)
E. Decrease the volume
-
Question 39:
Rank the freezing point in ascending order of the following solutions:
I. 2.0 m CH3OH
II. 2.0 m NaCl
III.
2.0 m AlCl3
A.
III < II < I
B.
I > II > III
C.
I > III > II
D.
II > I > III
E.
I = II = III
-
Question 40:
Which of the following phase changes leads to a positive change in entropy value?
A. Fusion
B. Melting
C. Sublimation
D. Vaporization
E. All of the above
Related Exams:
AACD
American Academy of Cosmetic DentistryACLS
Advanced Cardiac Life SupportASSET
ASSET Short Placement Tests Developed by ACTASSET-TEST
ASSET Short Placement Tests Developed by ACTBUSINESS-ENVIRONMENT-AND-CONCEPTS
Certified Public Accountant (Business Environment amd Concepts)CBEST-SECTION-1
California Basic Educational Skills Test - MathCBEST-SECTION-2
California Basic Educational Skills Test - ReadingCCE-CCC
Certified Cost Consultant / Cost Engineer (AACE International)CGFNS
Commission on Graduates of Foreign Nursing SchoolsCLEP-BUSINESS
CLEP Business: Financial Accounting, Business Law, Information Systems & Computer Applications, Management, Marketing
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Test Prep exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your OAT exam preparations and Test Prep certification application, do not hesitate to visit our Vcedump.com to find your solutions here.