Exam Details
Exam Code
:OATExam Name
:Optometry AdmissionCertification
:Test Prep CertificationsVendor
:Test PrepTotal Questions
:274 Q&AsLast Updated
:Aug 10, 2025
Test Prep Test Prep Certifications OAT Questions & Answers
-
Question 21:
Which of the following, when reacted with benzene, will direct an ortho/para configuration?
A. -COOH
B. -Cl
C. -CH3
D. NO2
E. 2 of the above
-
Question 22:
Which of the following is false regarding SN2 reactions?
A. SN2 occurs in one step.
B. Common solvents include DMSO and acetone.
C. Steric hindrance increases from primary to secondary to tertiary.
D. SN2 prefers to proceed with strong nucleophiles.
E. None of the above.
-
Question 23:
What is the relationship between the following two molecules?

A. Enantiomers
B. Identical compounds
C. Meso compounds
D. Diastereomers
E. Structural isomers
-
Question 24:
Identify the missing reagent in the reaction below:

A. 1) BH3, THF; 2) H2O2, OH
B. CH2N2, Heat
C. Cl2, CCl4
D. 1) OsO4; 2) NaHSO3
E. 1) O3; 2) Zn, H2O
-
Question 25:
Which of the following compounds is the weakest acid?
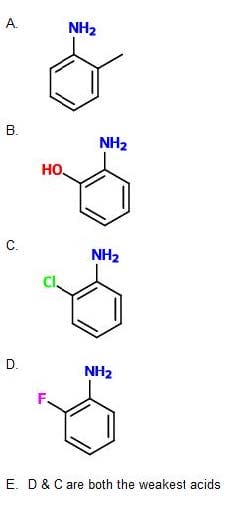
A. Option A
B. Option B
C. Option C
D. Option D
E. Option E
-
Question 26:
Identify the starting molecule in this reaction: A. Option A
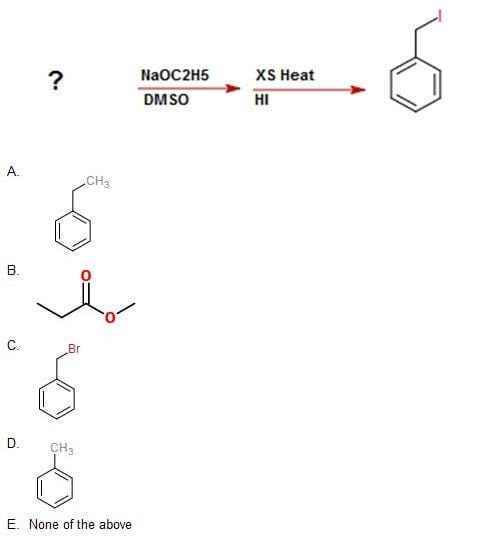
B. Option B
C. Option C
D. Option D
E. Option E
-
Question 27:
In IUPAC nomenclature, which of the following functional groups has the highest priority in numbering a parent chain?
A. Alkanes
B. Alkenes
C. Alkynes
D. Amines
E. Esters
-
Question 28:
Which of the following has the higher acidity?
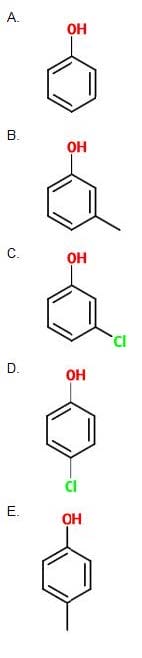
A. Option A
B. Option B
C. Option C
D. Option D
E. Option E
-
Question 29:
What is the percent composition of potassium in potassium dichromate?
A. 26.5%
B. 35.4%
C. 33.3%
D. 38.1%
E. Cannot be determined.
-
Question 30:
A vessel contains 0.50 moles of nitrogen, 0.40 moles of carbon and 0.10 moles of chlorine at a total pressure of 4.0 atm. What is the partial pressure of the chlorine?
A. 0.40 atm
B. 2.00 atm
C. 1.60 atm
D. 1.00 atm
E. Cannot be calculated with this information.
Related Exams:
AACD
American Academy of Cosmetic DentistryACLS
Advanced Cardiac Life SupportASSET
ASSET Short Placement Tests Developed by ACTASSET-TEST
ASSET Short Placement Tests Developed by ACTBUSINESS-ENVIRONMENT-AND-CONCEPTS
Certified Public Accountant (Business Environment amd Concepts)CBEST-SECTION-1
California Basic Educational Skills Test - MathCBEST-SECTION-2
California Basic Educational Skills Test - ReadingCCE-CCC
Certified Cost Consultant / Cost Engineer (AACE International)CGFNS
Commission on Graduates of Foreign Nursing SchoolsCLEP-BUSINESS
CLEP Business: Financial Accounting, Business Law, Information Systems & Computer Applications, Management, Marketing
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Test Prep exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your OAT exam preparations and Test Prep certification application, do not hesitate to visit our Vcedump.com to find your solutions here.