Exam Details
Exam Code
:A00-280Exam Name
:SAS Certified Clinical Trials Programmer Using SAS 9Certification
:SAS Institute CertificationsVendor
:SAS InstituteTotal Questions
:99 Q&AsLast Updated
:Aug 20, 2025
SAS Institute SAS Institute Certifications A00-280 Questions & Answers
-
Question 11:
Where would you store a value collected on a case report form but not defined in an SDTM domain?
A. RELREC
B. DM
C. SUPPQUAL
D. SC
-
Question 12:
Given the following data set:
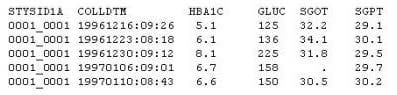
Which type of clinical trials data is this?
A. Laboratory
B. Baseline
C. Medical History
D. Vital Signs
-
Question 13:
Identify the CDISC model with the following characteristics:
?XML-based content and format standard
?facilitates the archive and interchange of the metadata and data for clinical research
?provides an accurate audit trail that is 21 CRF Part II compliant
A. Analysis Data Model (ADaM)
B. Operational Data Model (ODM)
C. Study Data Tabulation Model (SDTM)
D. Trial Design Model (TDM)
-
Question 14:
Which CDISC standard is concerned with the development of simplified case report forms?
A. Clinical Data Acquisition Standards Harmonization (CDASH)
B. Operational Data Model (ODM)
C. Study Data Tabulation Model (SDTM)
D. Trial Design Model (TDM)
-
Question 15:
Define.xml is an XML-based submission of a clinical study's: A. results
B. metadata
C. data
D. protocol
-
Question 16:
A subject reports a medication started in March of 2007 but cannot recall the day number. What is the value stored in the SDTM domain CM.CMSTDTC variable?
A. 00MAR2007
B. 2007 03
C. MAR2007
D. 2007-03
-
Question 17:
Given the following data set:
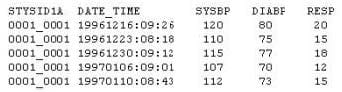
Which type of clinical trials data is this?
A. Demographics
B. Laboratory
C. Medical History
D. Vital Signs
-
Question 18:
Identify the data structure with the following characteristics:
?Contains one or more records per subject, per analysis parameter, and per analysis timepoint.
?May be derived from findings, events, interventions and special-purpose SDTM domains, or other ADaM datasets.
?A record can represent an observed, derived, or imputed value required for analysis.
A. General Data Structure (GDS)
B. Basic Data Structure (BDS)
C. Subject Level Analysis Data Set (ADSL)
D. Event Level Analysis Data Set (ADAE)
-
Question 19:
This question will ask you to provide a missing option.
The following program is submitted to create a transport file for multiple data sets:
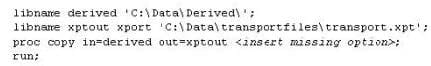
Which option will limit the files that are copied to type data?
A. intype=data
B. memtype=data
C. entrytype=data
D. include=data
-
Question 20:
The following SAS program is submitted:

Which alternative program creates an equivalent BASE_BP data set?
A. proc sort data=VS (keep=usubjid vsstresn vstestcd) out=BASE_BP (drop=vstestcd); where vstestcd in('DIABP','SYSBP'); by usubjid; run;
B. data BASE_BP; set VS (keep=usubjid vsstresn); if vstestcd in('DIABP','SYSBP'); run;
C. proc sort data=VS (keep=usubjid vsstresn vstestcd) out=BASE_BP (drop=vstestcd); by usubjid; if vstestcd in('DIABP','SYSBP'); run;
D. data BASE_BP (keep=usubjid vsstresn vstestcd); set VS (drop=vstestcd); if vstestcd in('DIABP','SYSBP'); run;
Related Exams:
A00-201
SAS Base ProgrammingA00-202
SAS Advanced ProgrammingA00-203
SAS Warehouse Development Specialist ConceptsA00-204
SAS Warehouse Architect ConceptsA00-206
SAS Warehouse TechnologyA00-211
SAS Base Programming for SAS 9A00-212
SAS Advanced Programming Exam for SAS 9A00-215
SAS 9.4 Programming FundamentalsA00-231
SAS 9.4 Base Programming - Performance-BasedA00-240
SAS Statistical Business Analysis Using SAS 9: Regression and Modeling
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only SAS Institute exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your A00-280 exam preparations and SAS Institute certification application, do not hesitate to visit our Vcedump.com to find your solutions here.